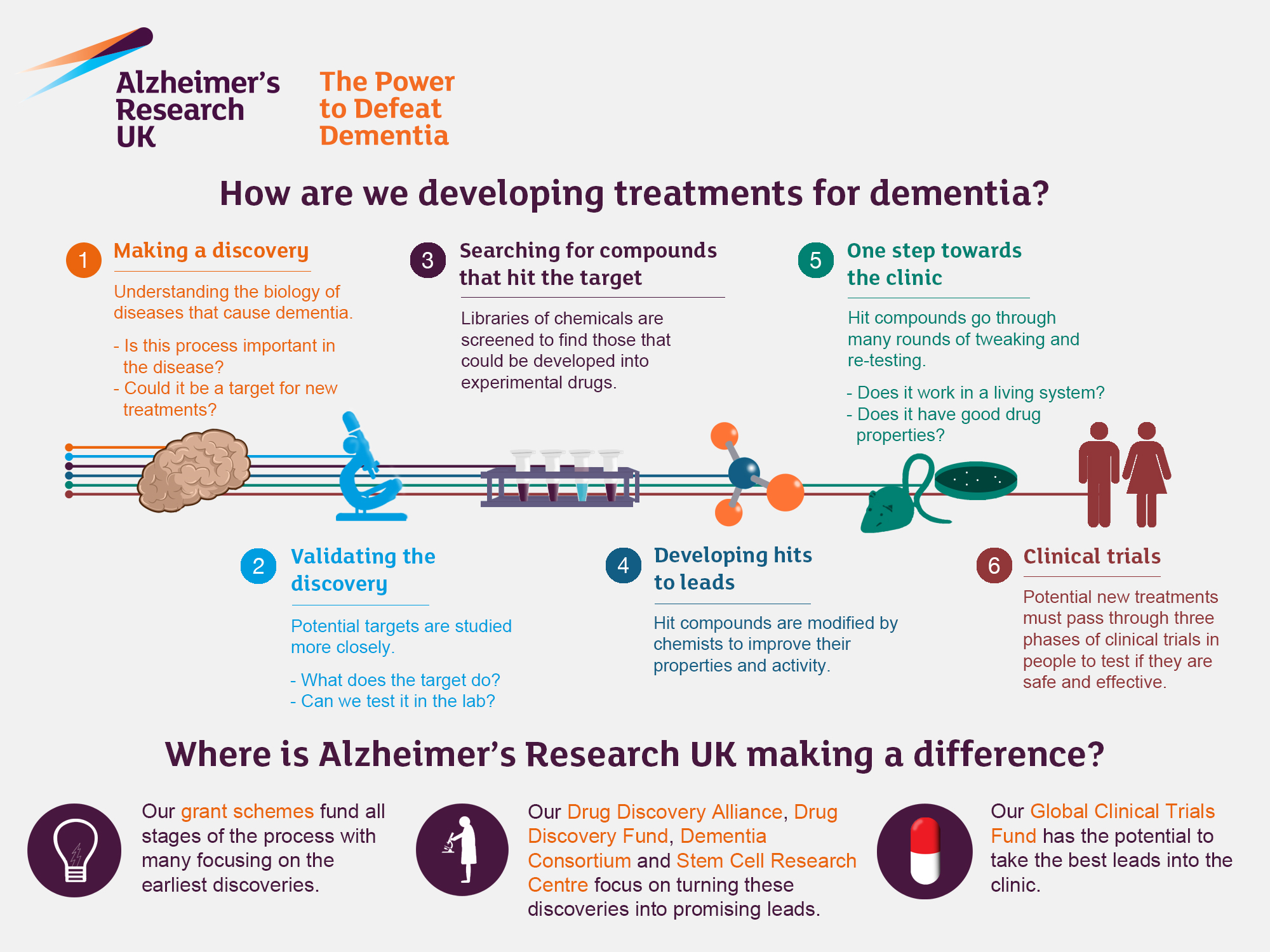
Alzheimer’s research has become a pivotal focus in neuroscience, especially with groundbreaking insights from scientists like Beth Stevens. This field delves deep into the role of microglial cells, which act as the brain’s immune system, tirelessly monitoring for damage and clearing out dead or faulty neurons. Recent studies have highlighted how these cells also engage in synaptic pruning—an essential process of refining neuronal connections that can inadvertently contribute to neurodegenerative diseases such as Alzheimer’s. Stevens and her team have unearthed significant findings that suggest aberrant synaptic pruning could exacerbate these conditions, opening doors for potential biomarkers and therapeutic strategies. As we grapple with the staggering number of individuals affected by Alzheimer’s in the U.S., her pioneering work not only aims to protect the brain but also to redefine our understanding of the disease itself.
Research into Alzheimer’s disease encompasses a variety of innovative approaches aimed at unraveling the complexities of this devastating condition. Often described as a leading cause of dementia, the exploration of neurodegenerative disorders involves studying brain function and the intricate interactions within the brain’s immune framework. Investigators like Beth Stevens focus on the behaviors of microglial cells, which are critical to maintaining brain health through their role in monitoring cellular activity and regulating synaptic connections. By examining neuroinflammation and the processes of synaptic remodeling, researchers are making strides in identifying new avenues for potential interventions. Understanding the dynamics of these processes not only sheds light on Alzheimer’s but also contributes to the broader landscape of neurodegenerative research.
Revolutionizing Alzheimer’s Research Through Microglial Cells
Alzheimer’s research has taken a transformative leap with new insights into the role of microglial cells, which act as the brain’s immune system. These specialized cells are responsible for monitoring and maintaining brain health by removing debris and guiding synaptic pruning. Through the pioneering work of researchers like Beth Stevens, the complex relationship between microglia and neurodegenerative diseases is becoming clearer. By understanding how these cells operate, scientists are poised to develop new biomarkers and therapeutic strategies aimed at slowing or potentially reversing the effects of Alzheimer’s disease.
The research led by Stevens’ lab at Boston Children’s Hospital has opened avenues for innovative treatments that could impact millions of lives, particularly for the estimated 7 million Americans diagnosed with Alzheimer’s. Aberrant synaptic pruning by microglia, as demonstrated in Stevens’ studies, highlights a potential target for intervention. This brings hope not only for Alzheimer’s but also for related neurodegenerative disorders like Huntington’s. As the way we perceive microglial function evolves, so too does our approach to tackling these challenging diseases.
Understanding the Brain’s Immune System: Microglia and Neurodegenerative Diseases
The brain’s immune system, primarily represented by microglial cells, plays a crucial role in safeguarding against neurodegenerative diseases. In conditions like Alzheimer’s, microglia can become overactive, leading to excessive synaptic pruning that disrupts communication between neurons. This cellular imbalance is a key factor in the progression of many neurodegenerative diseases, emphasizing the need for ongoing research in this area. By studying microglial behavior, scientists can uncover critical insights into how these cells can be regulated to restore healthy brain function.
Researchers like Beth Stevens are pioneering this field by exploring how microglial cells transition from protective functions to potentially harmful activities as disorders progress. Funding from organizations like the National Institutes of Health has facilitated this vital research, ultimately aiming to better understand how the brain’s immune responses can be manipulated for therapeutic gain. This knowledge not only lays the groundwork for new Alzheimer’s treatments but also enhances our understanding of the underlying processes that define neurodegenerative diseases.
The Importance of Synaptic Pruning in Brain Development
Synaptic pruning is a natural process that is crucial for healthy brain development. This mechanism involves the elimination of excess synapses to refine neural circuits, ensuring that only the most important connections are maintained. Microglial cells play a significant role in this process by identifying and removing less active or damaged synapses. While this pruning is beneficial during normal brain development, improper regulation of microglial activity can lead to neurological disorders such as Alzheimer’s disease, where the loss of synaptic connections disrupts cognitive function.
Beth Stevens’ work illustrates the delicate balance required for effective synaptic pruning and the repercussions of its dysregulation. Current research aims to delineate the conditions under which microglia engage in beneficial versus harmful pruning. By investigating the mechanisms behind synaptic remodeling, scientists hope to find therapeutic targets that can enhance synaptic health and potentially mitigate the cognitive decline associated with neurodegenerative diseases, ushering in a new era of brain health.
How Neurodegenerative Diseases Affect Brain Function
Neurodegenerative diseases like Alzheimer’s disease dramatically impact brain function, affecting memory, cognition, and overall quality of life. As these conditions progress, they lead to the gradual loss of neurons and synaptic connections, which are vital for effective communication within the brain. This loss of neuronal integrity can be partly attributed to the malfunctioning of microglial cells, which, instead of protecting the brain, may contribute to neuroinflammation and increased synaptic pruning.
Understanding the pathophysiology of neurodegenerative diseases is critical as it reveals potential therapeutic avenues. Research led by experts like Beth Stevens provides critical insights into how microglial cells modify synaptic networks during neurodegeneration. By elucidating the mechanisms of these diseases, we can aim for targeted therapies that restore cognitive function and halt disease progression. As this field evolves, the hope is to improve treatment strategies and enhance patient outcomes.
The Role of Beth Stevens in Neurological Research
Beth Stevens has emerged as a significant figure in neurological research, particularly in the exploration of the immune system’s role within the brain. Recognized as a MacArthur “genius,” her groundbreaking studies on microglial cells have reshaped our understanding of their functions in neurodevelopment and disease. By elucidating the mechanisms through which microglia prune synaptic connections, Stevens’ research is integral to developing potential interventions for Alzheimer’s disease and other neurodegenerative disorders.
Her dedication to understanding the basic science of brain immunity drives crucial discoveries that lead to clinical applications. Funding from the NIH and other institutions has allowed Stevens and her team to pursue innovative research, yielding insights that illuminate how inflammatory processes can influence neurodegenerative diseases. This research not only holds promise for Alzheimer’s care but also pushes the boundaries of our understanding of brain health at large.
Innovations in Biomarkers for Neurodegenerative Diseases
The development of biomarkers for neurodegenerative diseases represents a groundbreaking step in diagnosis and treatment strategies. As Beth Stevens’ research highlights, understanding the function of microglial cells can lead to the identification of specific biomarkers that reflect synaptic health and cognitive decline. These biomarkers could provide early detection of Alzheimer’s disease, allowing for timely intervention and personalized treatment plans that may slow disease progression.
Furthermore, the discovery of new biomarkers could facilitate the population-wide screening of at-risk individuals, contributing to a better understanding of neurodegenerative disease pathology. The advancements in this area underscore the value of integrating basic science with clinical research, as discoveries in cellular processes can directly impact patient management strategies. Stevens’ work not only represents a pivotal advancement in Alzheimer’s research but also presents hope in the ongoing battle against various neurodegenerative diseases.
The Future of Alzheimer’s Treatment and Research
As we look towards the future, Alzheimer’s treatment and research stand at a promising crossroads. The integration of findings related to microglial function and synaptic pruning is setting the stage for novel therapeutic approaches that could revolutionize care for those affected by this debilitating disease. With researchers like Beth Stevens paving the way, there is a renewed focus on targeting the underlying biological mechanisms rather than merely addressing the symptoms.
The exploration of microglial cells and their impact on brain health is paramount to finding effective therapies. As initiatives to fund basic and applied Alzheimer’s research continue, the hope is that new treatments will emerge that address the root causes of the disease. Innovations in scientific discovery, coupled with advances in technology, are expected to provide the tools needed to unlock new potentials in the fight against Alzheimer’s, ultimately leading to improved outcomes for millions.
Funding and Support in Alzheimer’s Research
The necessity for funding and support in Alzheimer’s research cannot be overstated. Significant contributions from entities like the National Institutes of Health (NIH) have enabled researchers such as Beth Stevens to pursue groundbreaking studies that may alter the landscape of neurodegenerative disease treatment. Grants facilitate rigorous research that leads to discoveries about microglial cells and their roles in synaptic pruning and Alzheimer’s pathology.
Increased investment in this area not only supports scientific exploration but also creates a framework for future advancements. As funding increases, so too does the potential for collaborative efforts that involve public health officials, scientists, and regulatory agencies. This synergy is essential for translating research discoveries into clinical practice, ensuring that innovations in Alzheimer’s detection and treatment effectively reach those in need.
The Impact of Neuroinflammation on Alzheimer’s Disease Progression
Neuroinflammation is a critical aspect of Alzheimer’s disease progression that has garnered increased attention in recent years. Microglial cells, while essential for maintaining brain health, can become overactive in response to neurodegenerative diseases, leading to chronic inflammation that aggravates neuronal damage. Understanding this relationship is crucial for devising strategies to modulate microglial activity and reduce neuroinflammatory responses associated with Alzheimer’s.
Research spearheaded by prominent scientists like Beth Stevens has illuminated the pathways through which inflammation contributes to cognitive decline. By targeting these inflammatory processes, novel therapeutic strategies could emerge, offering new hope for managing and potentially reversing the impacts of Alzheimer’s disease. As this field continues to grow, the importance of addressing neuroinflammation within the broader context of neurodegenerative diseases will remain a focal point.
Frequently Asked Questions
How do microglial cells contribute to Alzheimer’s research?
Microglial cells play a crucial role in Alzheimer’s research as they act as the brain’s immune system, clearing damaged cells and performing synaptic pruning. Research led by Beth Stevens has shown that abnormal activity of microglia can lead to the development of neurodegenerative diseases like Alzheimer’s, enlightening pathways for potential treatments.
What is synaptic pruning and its relevance in Alzheimer’s research?
Synaptic pruning is the process by which microglial cells eliminate unnecessary synapses to optimize neural networks. In Alzheimer’s research, it has been discovered that excessive or incorrect synaptic pruning can contribute to the disease, highlighting the importance of understanding microglial behavior for developing therapies.
What advancements have been made in Alzheimer’s research regarding neurodegenerative diseases?
Advancements in Alzheimer’s research have revealed that the dysfunction of microglial cells, responsible for maintaining brain health, can lead to neurodegenerative diseases. The work of researchers like Beth Stevens has laid the groundwork for new biomarkers and medications that target these immune responses in the brain.
How are Beth Stevens’ findings influencing Alzheimer’s disease treatments?
Beth Stevens’ findings on microglial cells and their role in synaptic pruning are influencing Alzheimer’s disease treatments by identifying how improper pruning relates to disease progression. This ongoing research aims to create novel therapies that can restore proper microglial functions and potentially halt the progression of Alzheimer’s.
What role does the brain’s immune system play in Alzheimer’s research?
The brain’s immune system, primarily consisting of microglial cells, is vital in Alzheimer’s research as it helps in maintaining neural health. Understanding how this immune system interacts with synaptic pruning is crucial for developing effective treatments and managing neurodegenerative diseases.
Why is the study of neurodegenerative diseases important in relation to Alzheimer’s research?
The study of neurodegenerative diseases is paramount in Alzheimer’s research because it helps uncover common mechanisms that underlie multiple disorders. Insights gained from understanding how microglial cells contribute to conditions like Alzheimer’s can lead to better prevention and treatment strategies.
| Key Point | Details |
|---|---|
| Role of Microglia | Microglia act as the brain’s immune system, clearing dead cells and pruning synapses. |
Summary
Alzheimer’s research is advancing rapidly, thanks to innovative work being done by scientists like Beth Stevens. Her research at Boston Children’s Hospital has unveiled crucial insights into the role of microglial cells in neurodegenerative diseases, particularly Alzheimer’s disease. By understanding how these immune cells respond to brain injuries and their role in synaptic pruning, new pathways for treatment are being developed. Stevens’ findings underscore the importance of federal support in basic science, which eventually leads to major breakthroughs in Alzheimer’s care and treatment. As we learn more about the intricacies of the brain’s immune system, the potential for developing effective interventions for the millions affected by Alzheimer’s continues to grow.